
NASA/JPL-Caltech
A new high-pressure ventilator developed by NASA engineers and designed to treat COVID-19 has received FDA approval via a fast-tracked emergency use authorization. Now,NASA is looking for a medical industry partner to manufacture the device. It will license the tech on a royalty-free basis during the pandemic.
Engineers from NASA’s Jet Propulsion Laboratory (JPL) developed VITAL (Ventilator Intervention Technology Accessible Locally) in just 37 days. It has been tested successfully on a “high fidelity human patient simulator” at the Icahn School of Medicine at Mount Sinai in New York.
"Now that we have a design,we're working to pass the baton to the medical community,and ultimately patients,as quickly as possible," said Fred Farina,chief innovation and corporate partnerships officer at Caltech.
The design offers a few key benefits. NASA says it can be built faster and maintained more easily than traditional ventilators. It is composed of fewer parts,which are currently available through existing supply chains,and it can be modified for use in field hospitals like the ones being set up in convention centers. The device is built to last three to four months,so it won’t replace the current hospital ventilators,but it could fill critical shortages.
JPL doesn’t typically design medical equipment,but in a video,several engineers expressed a desire to use their skills to help address the COVID-19 pandemic. This is part of a growing trend of technologists trying to meet the demand for ventilators. We’ve seen right-to-repair campaigns,as well as ventilators made with gaming PC cases and Tesla parts. We’ve also learned,though,that while making ventilators is relatively easy,pivoting manufacturing to do so is more difficult.
“This ventilator is one of countless examples of how taxpayer investments in space exploration — the skills,expertise and knowledge collected over decades of pushing boundaries and achieving firsts for humanity — translate into advancements that improve life on Earth,” NASA Administrator Jim Bridenstine said in a statement.









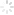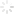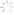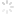 加载中,请稍侯......
加载中,请稍侯......
Comments